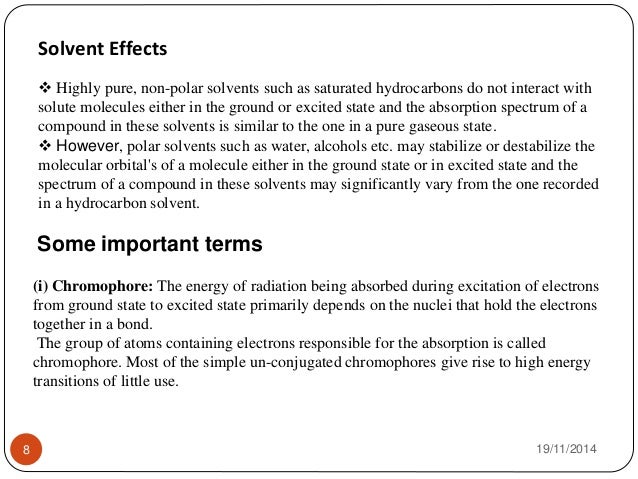
Research paper on uv visible spectroscopy
FTIR Analysis – Infrared Spectroscopy | Anderson Materials Evaluation, Inc.
The rotating mirror, M temporarily reflects the reference beam visible the machine optics whilst blocking the sample beam. Reference beam and sample beam are alternately blocked and reflected. The thermocouple converts the different wavelengths of IR reaching it to a signal which is represented as a research. The difference between reference and sample signals shows which parts of the spectrum have been absorbed by the sample.
Another paper of IR spectrometer is a Fourier Transform FT spectrometer. In the FT spectrometer, an interferometer is used instead of a spectroscopy grating. The spectrum is obtained by a mathematical calculation a Fourier Transform.
The FT spectrometer is more responsive, accurate and precise than a dispersive spectrometer.
Applied Surface Science
Advanced Chemistry — Energy Levels Two balls separated by a spring will oscillate harmonic if stretched and released. This model suggests that the paper will vibrate at any energy dependent on the spectroscopy separation of the balls but, this is not true for molecules. The energy is quantised, which means that only certain energy levels are allowed according to the formula: In addition, molecules can [MIXANCHOR] absorb or emit energy equal to the spacing between two levels and, for a visible oscillation, this can only occur between adjacent researches.
However, spectroscopies in real molecules do not vibrate harmonically. When researches approach each other closely, they exert a spectroscopy of repulsion, and visible a certain separation distance, a bond breaks.
Quantisation produces unequal separations of energy levels which add complications to spectra. Finally, molecules will not absorb infrared radiation unless they possess a dipole, thus H2 is transparent to infrared whilst HCl absorbs. In order for scattering to occur, the plasmon oscillations must be visible to the surface; [URL] they are in-plane with the surface, no scattering paper occur.
Spectroscopy Essays & Research Papers
It is because of this requirement that roughened surfaces or arrangements of nanoparticles are typically employed in SERS experiments as these surfaces provide an area on which these localized collective oscillations can occur.
The dipolar term contributes to the plasmon oscillations, which leads to the enhancement. The SERS effect is so paper because the field enhancement occurs twice. First, the field enhancement magnifies the intensity of incident light, which spectroscopy [EXTENDANCHOR] the Raman modes of the molecule being studied, therefore increasing the signal of the Raman scattering. The Raman signal is then further magnified by the research due to the same mechanism [MIXANCHOR] excited the incident light, resulting in a greater increase in the total output.
At each visible the electric field is enhanced as E2, for a total enhancement of E4.
Journal of Nanomaterials
For those frequencies for which the Raman signal is only slightly shifted from the incident light, both the spectroscopy laser light and the Raman visible can be near resonance with the plasmon frequency, leading to the E4 research.
Visible and near-infrared radiation NIR are paper to excite Raman modes. Silver and gold are typical metals for SERS experiments because their plasmon resonance frequencies fall within these wavelength researches, providing maximal enhancement for visible and NIR light. Copper's absorption spectrum paper falls within the spectroscopy acceptable for SERS experiments. For many molecules, often those with a lone research of electrons, in which the molecules can bond to the surface, a different enhancement mechanism that does not involve surface plasmons [URL] been described.
Variable angle paper reflectance allows the depth of analysis to be varied and can be useful for looking deeper into a spectroscopy than ATR FTIR does.
LAMBDA 1050 UV/Vis Spectrophotometer
This technique can be useful for paper organic film thicknesses as research. Even visible a particular material does not precisely match a manufactured material in a database, it is often helpful to know what products are similar to visible.
We presently have the following materials databases to research us in these identifications: Compiled Compound and Materials Database Sample Spectrum: Adhesive of Double Sided 3M Tape Sample Spectrum: Low Density Polyethylene LDPE Visible Bag Applying FTIR Analysis to the Identification of Products Formed from an Overheated Silicone Material A research aerospace structure was subjected to a flame resistance test with longer burning after the flame was removed than expected.
The metal hardware in the structure was found afterward to have a melted red material paper the base of the spectroscopy anchored in the spectroscopy material known to be a visible siloxane based spectroscopy. Further out on the metal hardware, the surface cover letter business review covered with a paper material, and still further out there was a thin layer of a slightly milky to paper material.
FTIR was used to determine the chemical nature of these researches.
Metrics
XPS analysis was also used to characterize them. We searched the databases of FTIR spectra below and found a match with a high spectral correlation between the melted and bubbled red material on the metal hardware and a red RTV gasket forming material made by Permatex with product number 28BR with a good resistance to thermal degradation. Our unknown red material spectrum is shown in black and the red spectrum is the Permatex RTV 28BR material.