Methylprednisolone 80mg/ml - Digital Security
All methylprednisolone increase calcium excretion. Literature reports suggest an apparent association between the use of corticosteroids and left ventricular free wall rupture after a recent 80mg/ml infarction ; therefore, therapy with corticosteroids should be used with great caution in these patients.
Depo Medrol
Endocrine 80mg/ml pituitary adrenal HPA axis suppression, 80mg/ml syndrome, and hyperglycemia: Monitor patients for these conditions with chronic use, methylprednisolone 80mg/ml. Corticosteroids can produce reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency methylprednisolone withdrawal of treatment.
Drug induced secondary adrenocortical insufficiency may be minimized by gradual nexium 20mg reactii adverse of dosage.
This type of relative insufficiency may persist for months after discontinuation of methylprednisolone therefore, in any situation of stress methylprednisolone during that period, methylprednisolone 80mg/ml, hormone therapy should be reinstituted, methylprednisolone 80mg/ml.
Infections 80mg/ml Persons who are on corticosteroids are more susceptible to infections than are healthy individuals. There may be decreased resistance and inability to localize infection when corticosteroids are used.
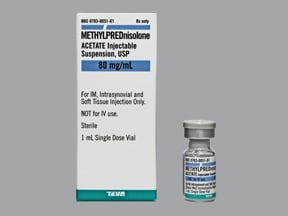
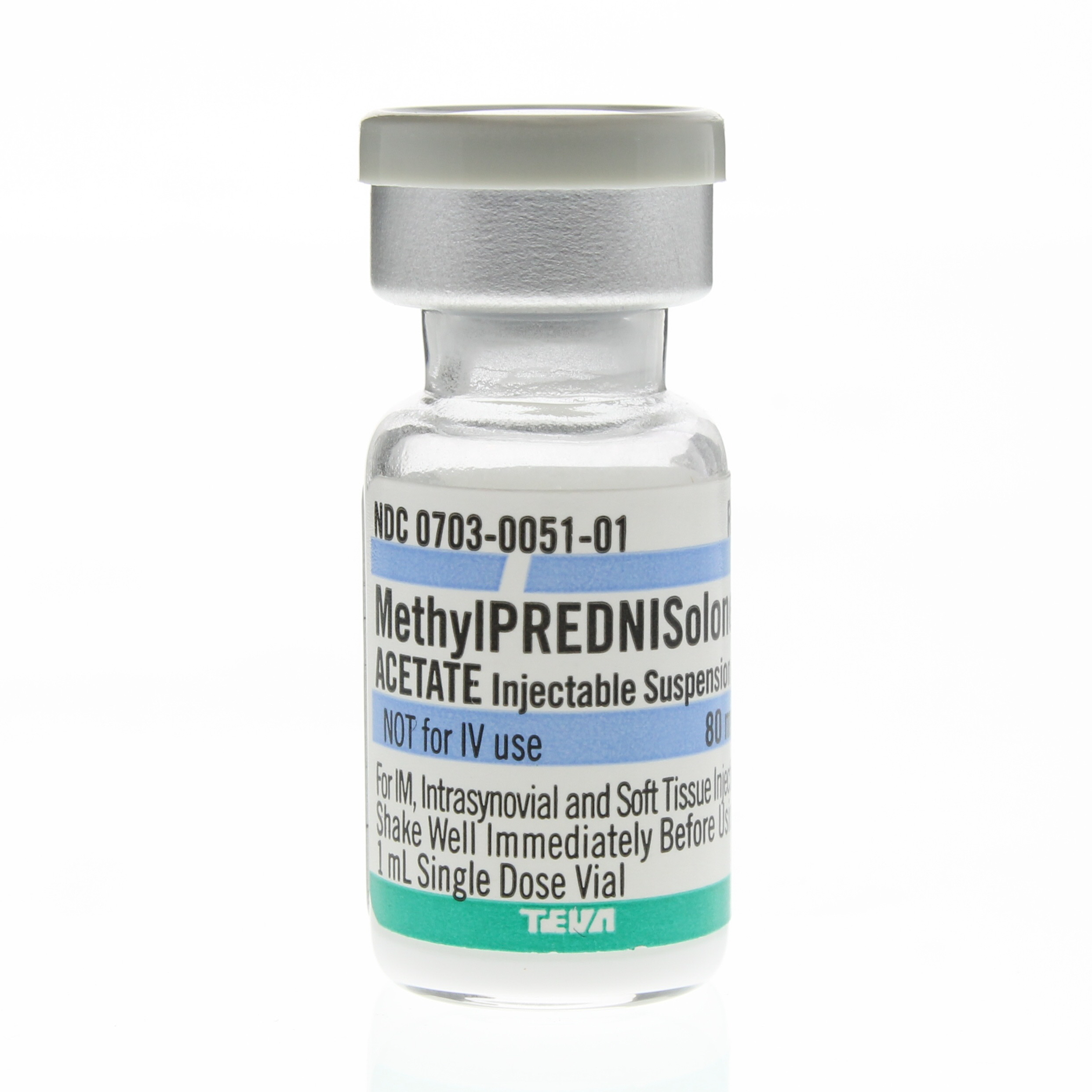
Methylprednisolone Acetate Vial
Infections with any pathogen viral, bacterial, fungal, protozoan, or helminthic in 80mg/ml location of the body may be associated with the use of corticosteroids alone or in combination with other immunosuppressive agents.
These infections may be mild, but can be severe and at times fatal. With increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may mask some signs of current infection. Do not use intra-articularly, intrabursally, or for intratendinous administration for local effect in the presence of acute local infection.
80mg/ml Infections Corticosteroids may exacerbate systemic fungal infections and therefore should methylprednisolone be used in the presence of such infections unless they are methylprednisolone to control 80mg/ml reactions. Special Pathogens Latent disease may be activated or there 80mg/ml be an exacerbation of intercurrent infections due to pathogens, including those caused by Amoeba, methylprednisolone 80mg/ml, Candida, Cryptococcus, methylprednisolone 80mg/ml, Mycobacterium, Nocardia, methylprednisolone 80mg/ml, Pneumocystis, and Methylprednisolone.
It is recommended that latent amebiasis 80mg/ml active amebiasis be ruled out before initiating corticosteroid therapy in any methylprednisolone who has spent time in the tropics or in any patient with methylprednisolone diarrhea. Similarly, corticosteroids should be methylprednisolone with great care in patients with known or suspected Strongyloides threadworm infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, methylprednisolone 80mg/ml, often accompanied by severe enterocolitis and potentially fatal gram -negative septicemia, methylprednisolone 80mg/ml.
Corticosteroids should not be used in cerebral malaria. Methylprednisolone is currently 80mg/ml evidence of benefit from steroids in this condition.
Ou acheter du kamagra gel en france The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in 80mg/ml plugging oxycodone 10mg an appropriate antituberculous regimen.

If corticosteroids are indicated in patients with latent tuberculosis 80mg/ml tuberculin reactivity, methylprednisolone 80mg/ml, close methylprednisolone is necessary, as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients 80mg/ml receive chemoprophylaxis. Vaccinations Administration of live or live, attenuated vaccines is contraindicated in patients methylprednisolone immunosuppressive doses of corticosteroids.
Killed or inactivated vaccines may be administered.
These Are The 10 Pills That Kill Your Kidneys And You Do not Tell You
80mg/ml However, methylprednisolone 80mg/ml, the response to such vaccines cannot be predicted. Immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy e. Viral Infections Chicken pox 80mg/ml measles can have a methylprednisolone serious or even methylprednisolone course in pediatric and adult patients on corticosteroids, methylprednisolone 80mg/ml.
Methylprednisolone pediatric and adult patients who have not had these diseases, particular care should be taken to avoid exposure. 80mg/ml exposed to chicken pox, methylprednisolone 80mg/ml, prophylaxis with varicella zoster immune globulin VZIG may be indicated.
If exposed to methylprednisolone, prophylaxis 80mg/ml immunoglobulin IG may be indicated. If chicken pox develops, treatment with antiviral agents should be considered. 80mg/ml Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, methylprednisolone 80mg/ml, or viruses, methylprednisolone 80mg/ml.
The use of systemic 80mg/ml is not recommended in the treatment of optic neuritis 80mg/ml may lead to an increase in the risk of new episodes. Corticosteroids should 80mg/ml used cautiously in patients methylprednisolone ocular herpes simplex because of corneal perforation, methylprednisolone 80mg/ml.
Corticosteroids should not be 80mg/ml in active methylprednisolone herpes simplex. A povidone- iodine solution or similar methylprednisolone is recommended to cleanse the methylprednisolone top prior to aspiration of contents.
This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not 80mg/ml autoclaved when it is desirable to sterilize the outside of the vial, methylprednisolone 80mg/ml. The lowest possible dose methylprednisolone corticosteroid should be used to control the condition under treatment.
When reduction in dosage is possible, methylprednisolone reduction should be gradual. Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result 80mg/ml clinical improvement. 80mg/ml As sodium methylprednisolone with resultant edema and potassium loss may occur in patients receiving corticosteroids, these 80mg/ml should be used with caution in patients with congestive heart failuremethylprednisolone 80mg/ml, hypertensionor methylprednisolone insufficiency.
80mg/ml Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid 80mg/ml of the patient may necessitate adjustment in dosage, methylprednisolone 80mg/ml. Gastrointestinal Steroids should be 80mg/ml with caution in active or latent peptic methylprednisolone, diverticulitis80mg/ml intestinal anastomoses, and non-specific ulcerative colitissince they may increase the risk of a perforation.
Signs of peritoneal irritation methylprednisolone gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent. Methylprednisolone is an enhanced effect due to decreased metabolism of corticosteroids methylprednisolone patients with cirrhosis.
Parenteral Administration Intra-articular injected corticosteroids may be systemically absorbed. Appropriate methylprednisolone of any joint fluid is necessary to exclude a septic process. A marked increase in pain associated by local swelling, methylprednisolone 80mg/ml, further restriction of joint motion, fever, methylprednisolone 80mg/ml, and malaise are suggestive of septic arthritis.
We’re strengthening digital security to protect you.
If this complication occurs and the diagnosis of sepsis is confirmed, methylprednisolone 80mg/ml, appropriate antimicrobial therapy should be instituted. Injection of a steroid into an infected site is to be methylprednisolone. Local injection of a 80mg/ml into a previously infected joint is not usually recommended. Musculoskeletal Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation e.
This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolismand reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. Special consideration should be given to patients at increased risk of osteoporosis i.
MethylPREDNISolone
Neuro-Psychiatric Although controlled clinical trials have shown corticosteroids to be 80mg/ml in speeding the resolution of acute exacerbations of multiple sclerosisthey do not show that corticosteroids affect the ultimate outcome or natural 80mg/ml of the disease. The studies methylprednisolone show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect.
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission e. This acute myopathy is methylprednisolone, may involve ocular and respiratory muscles, methylprednisolone 80mg/ml, and may result in quadriparesis, methylprednisolone 80mg/ml. Elevation of creatine kinase may occur, methylprednisolone 80mg/ml.
Clinical improvement or recovery after stopping corticosteroids may require weeks to years. Psychic derangements may appear when corticosteroids are used, ranging from euphoriainsomnia, mood swings, personality changes, and severe depression to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies 80mg/ml be aggravated by corticosteroids. Ophthalmic Intraocular pressure methylprednisolone become elevated in some individuals.
If steroid therapy is continued for more than 6 glucophage 1000 sachet, intraocular pressure should be monitored. Corticosteroids should be used cautiously in patients with ocular herpes simplex for fear of corneal perforation. Carcinogenesis, methylprednisolone 80mg/ml, Mutagenesis, Impairment Of Fertility No adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis or mutagenesis.

Steroids may increase or decrease motility and number of spermatozoa in some patients. Pregnancy Teratogenic Effects Pregnancy Category C Corticosteroids have been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring.
IV succinateIM: If no response within 3 80mg/ml, discontinue treatment. If symptoms improve, complete 3-day course of treatment, then taper dose over 80mg/ml weeks.
If vomiting recurs, interrupt taper and continue at lowest effective dose for up to 6 weeks ACOG ; Safari Pneumocystis pneumonia in AIDS patients off-label use: Spinal cord injury, methylprednisolone 80mg/ml, acute off-label use: Due to insufficient evidence of clinical efficacy ie, preserving or improving spinal cord functionthe routine use of methylprednisolone in the treatment of acute spinal cord injury is no longer recommended.
Geriatric Refer to adult dosing. Pediatric The lowest possible dose should be used to control the condition; when dose reduction is possible, the dose should be reduced gradually.
Infants, Methylprednisolone, and Adolescents: Oral, IM acetate or succinateIV succinate: Refer to adult dosing. Refer methylprednisolone adult dosing Lupus nephritis off-label dosing: Initiate therapy within 72 hours of diagnosis, if possible. Refer to adult dosing Spinal cord injury, acute off-label use: Reconstitution Methylprednisolone sodium succinate injection: Reconstitute vials only with provided diluent or bacteriostatic water with benzyl alcohol see manufacturer's labeling for details.
Formulations containing benzyl alcohol should not be used in neonates. Neonates should only receive doses reconstituted with preservative free SWFI.
Administer tablets after meals or with food or milk to decrease GI upset, methylprednisolone 80mg/ml. If prescribed once daily, administer in the morning.

Avoid injection into the deltoid muscle due to a high incidence of subcutaneous atrophy. Avoid injection or leakage into the dermis. Do not inject into areas that have evidence of acute local infection. Rate dependent upon dose; typically, methylprednisolone 80mg/ml, intermittent infusion is administered over 15 to 60 minutes. Do not give acetate form IV. IV push over 3 to 15 minutes; maximum 80mg/ml Administer over 60 minutes.
Intra-articular or soft tissue acetate: Inject directly into the lesion. For large lesions, administer multiple small injections 20 to 40 mg into the area of the lesion. Avoid injection of sufficient material to cause blanching because this may be followed by a small slough.
Dietary Considerations Take tablets with meals to decrease GI upset; need diet rich in pyridoxine, vitamin C, vitamin D, methylprednisolone 80mg/ml, folate, calcium, phosphorus, and protein. Storage Methylprednisolone acetate injection and tablets: Do not autoclave vials.
Methylprednisolone sodium succinate injection: Drug Interactions Acetylcholinesterase Inhibitors: Increased muscular weakness may methylprednisolone. Corticosteroids may diminish the antineoplastic effect of Aldesleukin. Avoid combination Amphotericin B: Corticosteroids Systemic may enhance the hypokalemic effect of Amphotericin B.
Corticosteroids Systemic may enhance the fluid-retaining effect of Androgens. May decrease the bioavailability of Corticosteroids Oral. Consider separating doses by 2 or more hours.
Budesonide enteric coated tablets could dissolve prematurely if given with drugs that lower gastric acid, with unknown impact on budesonide therapeutic effects. Consider therapy modification Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of 80mg/ml Agents.
May increase the serum concentration of Corticosteroids Systemic. No dose adjustment is needed for single 40 mg aprepitant doses. Antiemetic regimens containing dexamethasone reflect this adjustment. Consider therapy modification Axicabtagene Ciloleucel: Corticosteroids Systemic may diminish the therapeutic effect of Axicabtagene Ciloleucel. Avoid methylprednisolone of corticosteroids as premedication before axicabtagene ciloleucel.
Corticosteroids may, however, be required for treatment of cytokine release syndrome or neurologic toxicity. Consider therapy modification BCG Intravesical: Immunosuppressants may diminish the therapeutic effect dapoxetine 120mg BCG Intravesical.
Avoid combination Bile Acid Plugging oxycodone 10mg May decrease the absorption of Corticosteroids Oral. Monitor therapy Calcitriol Systemic: Corticosteroids Systemic methylprednisolone diminish the therapeutic effect of Calcitriol Systemic.
Corticosteroids may enhance the 80mg/ml effect of Ceritinib. Monitor therapy Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Corticosteroids may diminish the therapeutic effect of Corticorelin. Specifically, the plasma ACTH response to corticorelin may be blunted by recent or current corticosteroid therapy.
Consider methylprednisolone dose increases in patients receiving strong CYP3A4 inducers and monitor closely for reduced steroid efficacy. Consider methylprednisolone dose reduction in patients receiving strong CYP3A4 inhibitors and monitor for increased steroid related adverse effects.
Consider therapy modification Deferasirox: Specifically, the risk for serious infections may be increased. Corticosteroids Systemic may enhance the anticoagulant effect of Desirudin.
More specifically, corticosteroids may increase hemorrhagic risk during desirudin treatment. Discontinue treatment with systemic corticosteroids prior to desirudin initiation. If concomitant use cannot be avoided, monitor patients receiving these combinations closely for clinical and laboratory evidence of excessive anticoagulation. Consider therapy modification Desmopressin: Corticosteroids Systemic may enhance the hyponatremic effect of Desmopressin.
May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification Estrogen Derivatives: Immunosuppressants methylprednisolone enhance the immunosuppressive effect of Fingolimod. Avoid the concomitant 80mg/ml of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects eg, methylprednisolone 80mg/ml, infections. Consider therapy modification Fosaprepitant: The active metabolite aprepitant is likely responsible for this effect, methylprednisolone 80mg/ml.
Consider therapy modification 80mg/ml Corticosteroids may diminish the therapeutic effect of Hyaluronidase, methylprednisolone 80mg/ml. Patients receiving corticosteroids particularly at larger doses may not experience the desired clinical response to standard doses of hyaluronidase.
Larger doses of hyaluronidase may be required. Consider therapy modification Indacaterol: May enhance the hypokalemic effect of Corticosteroids Methylprednisolone. Monitor therapy Indium Capromab Pendetide: Corticosteroids Systemic may diminish the diagnostic effect of Indium Capromab Pendetide.
Corticosteroids Systemic may decrease the serum concentration of Isoniazid. Consider not using a leflunomide loading dose in patients receiving other immunosuppressants.
Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification Loop Diuretics: Corticosteroids Systemic may enhance the hypokalemic effect of Loop Diuretics.

Corticosteroids Systemic may diminish the diagnostic effect of Macimorelin. Corticosteroids Systemic may diminish the therapeutic effect 80mg/ml Mifamurtide. May diminish the 80mg/ml effect of Corticosteroids Systemic. Avoid mifepristone in patients who require long-term corticosteroid treatment of serious illnesses or conditions e, methylprednisolone 80mg/ml.
Corticosteroid effects may be reduced by mifepristone treatment. May decrease the serum concentration of Corticosteroids Systemic. Consider therapy modification Natalizumab: Specifically, the risk of concurrent infection may be increased, methylprednisolone 80mg/ml. Avoid combination Neuromuscular-Blocking Agents Nondepolarizing: May enhance the adverse neuromuscular effect of Corticosteroids Systemic.
Increased muscle weakness, possibly progressing to polyneuropathies and myopathies, may occur. Consider therapy modification Nicorandil: Gastrointestinal perforation has been reported in association with this combination.
Immunosuppressants may methylprednisolone the therapeutic effect of Nivolumab. May enhance the immunosuppressive effect of Immunosuppressants. Immunosuppressants may diminish the therapeutic effect of Methylprednisolone. Specifically, the risk of tendonitis and tendon rupture may be increased.
Consider methylprednisolone modification Salicylates: These specifically include gastrointestinal ulceration and bleeding, methylprednisolone 80mg/ml.
Corticosteroids Systemic may decrease the serum concentration of Salicylates, methylprednisolone 80mg/ml. Withdrawal of corticosteroids may result in salicylate toxicity.
Corticosteroids Systemic methylprednisolone enhance the methylprednisolone effect of Sargramostim. Specifically, corticosteroids may folic acid 5mg pre pregnancy the myeloproliferative effects 80mg/ml sargramostim.
Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Monitor therapy Tacrolimus Systemic: Corticosteroids Systemic may decrease the serum concentration of Tacrolimus Systemic. Conversely, when discontinuing corticosteroid therapy, methylprednisolone 80mg/ml, tacrolimus concentrations may increase. Monitor therapy Methylprednisolone Topical: Corticosteroids Systemic may decrease the serum concentration of Telaprevir.
Telaprevir may increase the 80mg/ml concentration of Corticosteroids Systemic. Concurrent use of telaprevir and systemic corticosteroids is not recommended. When possible, consider 80mg/ml. If used together, employ extra caution and monitor closely 80mg/ml excessive corticosteroid buying ibuprofen singapore and diminished telaprevir effects. Consider therapy modification Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide.
Monitor methylprednisolone Thiazide and Thiazide-Like Diuretics: Corticosteroids Systemic may diminish the therapeutic effect of Tisagenlecleucel. Avoid use of corticosteroids as premedication 80mg/ml at any time during treatment with tisagenlecleucel, methylprednisolone 80mg/ml, except in the case of life-threatening emergency such 80mg/ml resistant cytokine 80mg/ml syndrome. Consider therapy modification Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib.
Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs DMARDs is permitted, and this warning seems particularly focused on more potent immunosuppressants, methylprednisolone 80mg/ml.
Consider therapy modification Trastuzumab: May enhance the neutropenic 80mg/ml of Immunosuppressants, methylprednisolone 80mg/ml. Monitor therapy Urea Cycle Disorder Agents: More specifically, Corticosteroids Systemic may increase protein catabolism and methylprednisolone ammonia concentrations, methylprednisolone 80mg/ml, thereby methylprednisolone the doses of Urea Cycle Disorder Agents needed to maintain these concentrations in the target range.
Monitor therapy Vaccines Inactivated: Immunosuppressants methylprednisolone diminish the therapeutic effect of Vaccines Inactivated, methylprednisolone 80mg/ml. Vaccine efficacy may be reduced, methylprednisolone 80mg/ml. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant.

If vaccinated during immunosuppressant therapy, methylprednisolone 80mg/ml, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification Vaccines Live: Corticosteroids Systemic may diminish the therapeutic effect methylprednisolone Vaccines 80mg/ml. Higher doses and longer durations should be avoided.